Types of nutrients
Nutrients are classified into two large groups: organic and inorganic. Organic nutrients make up between 90% and 95% of the dry weight of plants and consist of the elements carbon, oxygen and hydrogen, from carbon dioxide in the air and water from the soil. The remaining 5 – 10% comprises what is called mineral fraction.
The beneficial effect of adding minerals to the soil, such as ash from fireplaces, and lime in rainy climates, has been known for centuries. However, until around 150 years ago, the function of the various mineral elements in plant growth was a subject of debate.
Until methods for analysing soils and vegetable tissue were developed, the earliest theories that accounted for the role of these different nutrients and how some of them are vital for plant growth, could not be properly formulated. The result was the notion of essential elements for plant growth.
One of the theories that emerged from these studies was the law of the minimum, or Liebig’s law (named after the German baron and chemist Julius von Liebig), in the mid-19th century. According to this theory, plant growth relies on various factors (water, CO2, nitrogen, light, relative humidity). In the absence of a minimum of these, plants stop producing biomass.
Later, in 1880, the botanist Julius von Sachs showed how plants could be grown in a nutrient solution without the need for any soil (hydroponics). Based on his experiments with hydroponic cultivation and his subsequent analysis of tissues, he came to the conclusion that neither the presence nor concentration of an element in a plant meant that it was essential.
Plants are not fully selective when absorbing nutrients, so may poison themselves if there are harmful elements in the soil or nutrient solution. They also absorb harmless, yet superfluous, elements.
Essential mineral elements
The term essential mineral element was coined by the American researchers Daniel Arnon and Arlow Scout in 1934. They argued that for an element to be considered essential it must meet the following three criteria:
- In its absence, the plant will be unable to complete its life cycle.
- The element’s role cannot be fulfilled by any other element.
- The element must be directly involved in metabolism, or be necessary for a specific metabolic phase, such as, for instance, an enzyme reaction.
If an element carries out some of the functions of an essential element or mitigates the effects of a harmful element, it may be referred to as a beneficial element.
For higher plants, such as the various cannabis species, seventeen essential elements have been identified, which are classified as either micro nutrients or macro nutrients. As their name suggests, micro nutrients are only necessary for some enzyme reactions. Even so, without them, the plant cannot complete its life cycle.
Some elements, like nickel, sodium and silicon, are only essential for a limited number of species, while in others they are considered beneficial.
| Classification | Element (only inorganic elements) |
| Macroelements | nitrogen, phosphorus, potassium, sulphur, magnesium, calcium |
| Microelements | iron, manganese, zinc, copper, boron, molybdenum |
| Beneficial elements | sodium, silicon, cobalt, iodine, vanadium, etc. |
As new methods for analysing tissues and the production of compounds are developed, the unintended addition of impurities will gradually be reduced, such that perhaps, this list will soon grow. Elements previously not considered beneficial could be added to fertiliser mixtures as impurities of the main compounds (especially in the case of microelements).
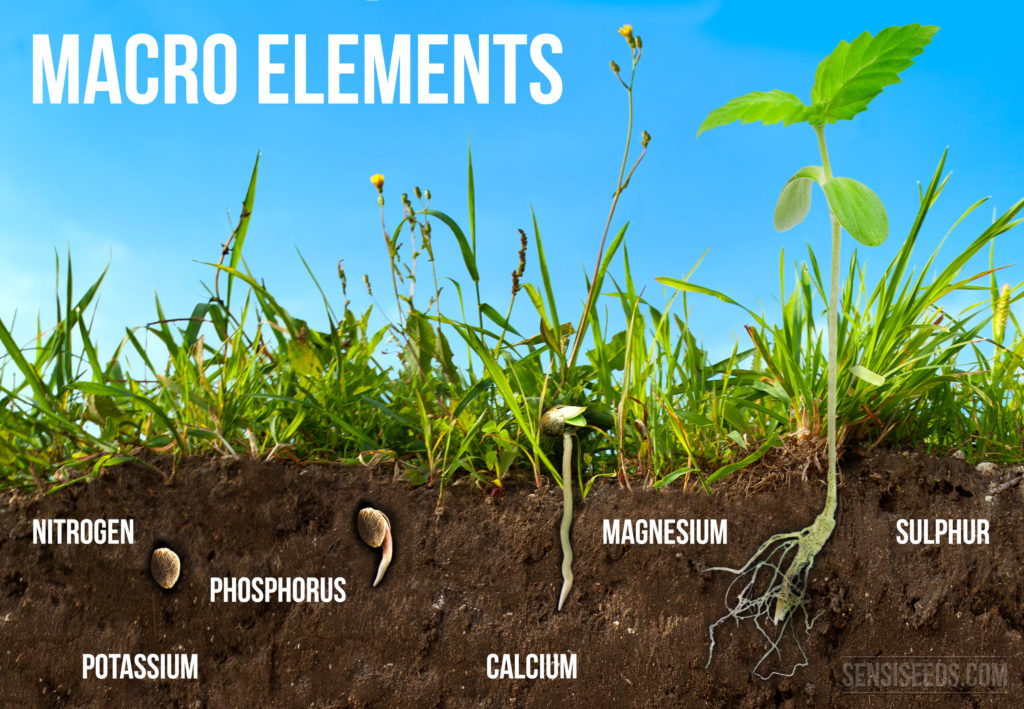
Macroelements

The six elements that we will address next (N, P, K, Mg, S, Ca), along with the organic elements carbon, hydrogen and oxygen, are all macroelements. Many of them are important to the structure of molecules and their name refers to the fact to that they are needed in large quantities (their content in tissues is always above 0.1%).
Nitrogen
After water, nitrogen is the most important nutrient for plant growth, as it is essential to the structure of proteins. In addition, the fact that the amount of nitrogen in most soils is very low, even in organic soils, means that nitrogen deficiency is very common, as is phosphorus and potassium deficiency.
Most nitrogen in soils is in its organic form, which plants cannot assimilate. Plants absorb nitrate, NO3–, and also ammonium, NH4+, from the soil. They can also absorb small amounts of ammonia gas through their leaves.
Another type of nitrogen acquisition can be observed in the leguminosae family. The roots of these plants live in symbiosis with certain soil bacteria capable of binding to atmospheric nitrogen gas, N2, which it then supplies to the plant. The plant then reduces or oxidises it to its ammonium or nitrate forms, respectively. Availability depends to a large extent on microbial life, which transforms organic forms into assimilable forms through a process called mineralisation.
This is why, in order to facilitate plant growth, it is vitally important to maintain a constant level of nitrogen in soils. The total nitrogen content in a plant’s dry weight varies between 1.5% and 5%.
Phosphorus
Phosphorus is assimilable by plants in its ion phosphate form, preferably absorbed as H2PO4– in neutral or slightly acidic soils and as HPO42- in chalky soils. Phosphorus is found in plants as phosphates in high-energy ATP (adenosine triphosphate) molecules, and plays a fundamental role in energy metabolic processes, respiration and photosynthesis.
In very chalky soils, phosphorus is not soluble and this necessitates administering it as a nutrient solution (for example, with a P-K 13-14). Mycorrhizal absorption is another important absorption route. Here, soil fungi solubilise and absorb phosphorus, so that its availability increases enormously, which benefits plant growth.
Potassium
Potassium is the main content of the most widely sold fertilisers. Its behaviour is very similar to that of nitrogen and phosphorus. It is easily diverted from the oldest to the youngest plant organs, which is why potassium deficiency is mainly observed in old or lower leaves. As it regulates the opening and closure of stomata, potassium plays a fundamental role in transpiration.
In addition, it activates more than fifty enzyme complexes, although in some cases it can be replaced by sodium. It is also responsible for the turgid appearance of plants (i.e. stiff and vigorous leaves and stems). K+ is the most abundant cation in plants and can comprise up to 10% of their dry weight.
Sulphur
Sulphur is found in some amino acids and various enzymes. It likewise plays an important role in cellular respiration. Sulphur is absorbed as sulphate, SO42-, and in the same form it is transported by the plant’s xylem. It can also be absorbed by leaf stomata as sulphur dioxide, SO2, an atmospheric pollutant produced by burning fossil fuels.
When this happens, a product called bisulphite is produced in the plant which displaces the magnesium from the chlorophyll molecule, thereby reducing photosynthesis. Often this is compensated for as, in places with a lot of traffic, carbon dioxide concentrations are also very high, which makes up for the adverse effects of gaseous sulphur. The sulphur-to-nitrogen ratio in plants is often 1:15.
Calcium
Calcium forms part of the cellular walls as calcium pectate and provides strength, just as it does in animal bones. It is also involved in plants’ adaptive mechanisms to light and temperature. Calcium is absorbed as the divalent ion Ca2+, which is abundant in most soils. Deficiency in this element rarely occurs, except if the plant is cultivated on untreated peat, which has a very acidic pH level.
From time immemorial, lime has been added to acidic soils in order to facilitate cultivation, so the essential nature of calcium has long been known. In hydroponic cultivation, with reverse osmosis water, calcium deficiency can result in poor root growth. In plants, calcium is found in concentrations of around 1% of dry matter.
Magnesium
Magnesium forms part of the chlorophyll molecule and is fundamental to successful plant growth. It is also involved in energy metabolism, forming compounds with ATP. It is absorbed as the divalent cation Mg2+ and its presence in soils is usually sufficient, except in very sandy or acidic soils.
Magnesium acts as a highly mobile element throughout the plant and for this reason, magnesium deficiency is always found in old leaves and their inter-nerval spaces.
- Disclaimer:Laws and regulations regarding cannabis cultivation differ from country to country. Sensi Seeds therefore strongly advises you to check your local laws and regulations. Do not act in conflict with the law.











hi,was reading your posting & was wondering if you had any more info on that meteorite product & if there are any ill / bad side effects from adding it? thnx
Good morning 420pati,
Thanks so much for your comment,
Unfortunately, I don’t have anymore information on any meteorite products. Maybe our readers know more?
Have a fantastic day!
Mark
This was a great discussion, thank you!
There needs to be more discussion on this topic because I found it to be very interesting and informative.
Good afternoon Berty,
Thanks for your comment and your feedback.
I’m so pleased you found the article to be interesting, there is definitely so much information to take in!
These articles on How to Spot the 7 Most Common Cannabis Plant Deficiencies by Leaf Symptoms and Beginners guide to home-made fertilizer might also be of interest to you.
Thanks again, and I hope you continue to enjoy the blog.
With best wishes,
Mark
I recently discovered a new soil amendment that produces extremely potent enough to blow your mind in one hit, Here’s the secret,I added1 gram of meteorite powder to each cubic foot of soil in the middle of the planter!Heres the title of the article Biological potential of extraterrestrial materials microbial and plant responsive to nutrient s in the murchinson carbonaceous meteorite, I have found a meteorite of the same type the article show s it shows the meteorite powder enhances plant greatl I call the plant Triptonite because it makes you trip this powder is very potent meteormike
amazing post ! Thanks for sharing.
Thank you for sharing such useful information.