Tetrahydrocannabinol (THC) is the most famous of all the cannabinoids by far, and is the principle psychoactive compound found to naturally occur in the cannabis plant. But what actually is the THC molecule, what is its purpose in nature, and what does it do in the human body?
Important questions about the nature and action of THC are being intensively studied by scientists the world over, and the results gained in the fifty years since the THC molecule was first discovered have offered untold benefit to medical science. As the research continues to intensify, benefits just keep stacking up and global barriers to understanding continue to be dismantled.
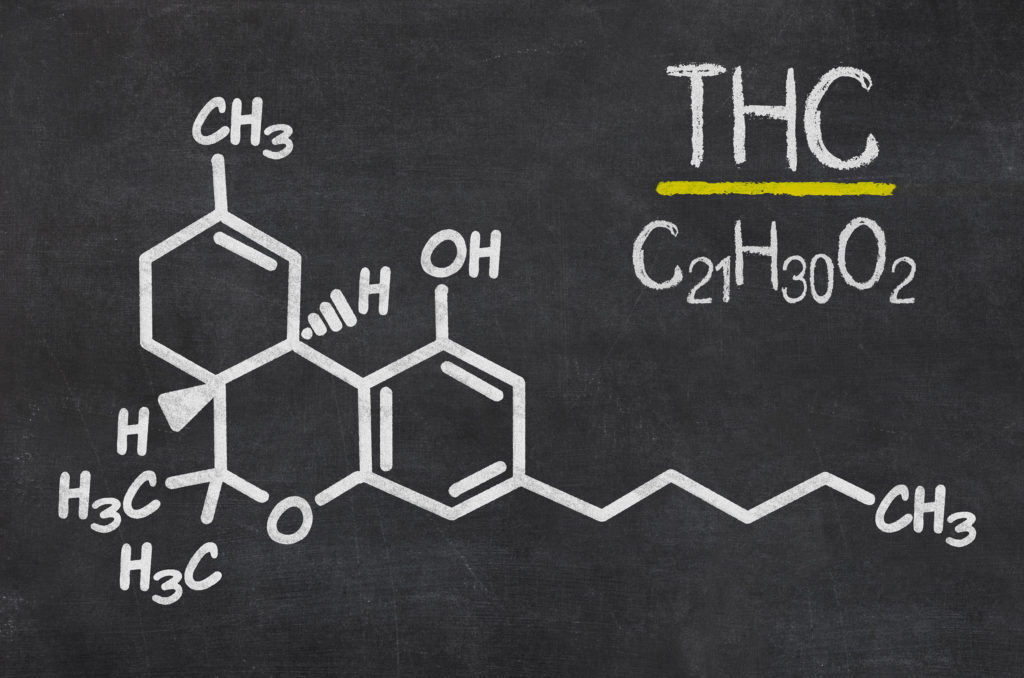
Chemical structure and properties of the THC molecule
THC is a molecule with the chemical formula C21H30O2, containing twenty-one atoms of carbon, thirty of hydrogen and two of oxygen. As with all other known phytocannabinoids, THC is an oily compound that is insoluble in water but highly soluble in lipid-based solvents.
Phytocannabinoids including THC are classed as terpenophenolic compounds. Terpenophenolics are complex organic molecules that comprise elements of both terpenoids and phenols (two major classes of naturally-occurring chemical compounds).
A complex chain reaction occurring in the leaves and resin glands of the cannabis plant leads to the production of THC. In the young leaves, a phenol known as olivetolic acid reacts with a compound known as geranyl pyrophosphate to form cannabigerolic acid.
How is THC produced by the cannabis plant?
Cannabigerolic acid (CBGA) is the precursor to many important cannabinoids, including THC, CBD, CBC, and CBG itself. To produce THC, CBGA must first undergo a reaction to form THC’s immediate precursor, known as tetrahydrocannabinolic acid (THCA). This reaction is facilitated by an enzyme known as THCA synthase.
The reaction takes place in the resin glands, ensuring that they are full of THCA. Then, in response to warm temperatures or simply by degradation over time, THCA is converted to THC. This process, whereby THCA becomes THC, is known as decarboxylation.
Quite simply, the THCA molecule is identical to the THC molecule with the addition of a “carboxyl group”; a simple arrangement of one carbon atom, one hydrogen atom and two oxygen atoms. When decarboxylation occurs, the carboxyl group is lost. This process also occurs with the other cannabinoid acids (such as CBCA and CBDA) to form the cannabinoids themselves.
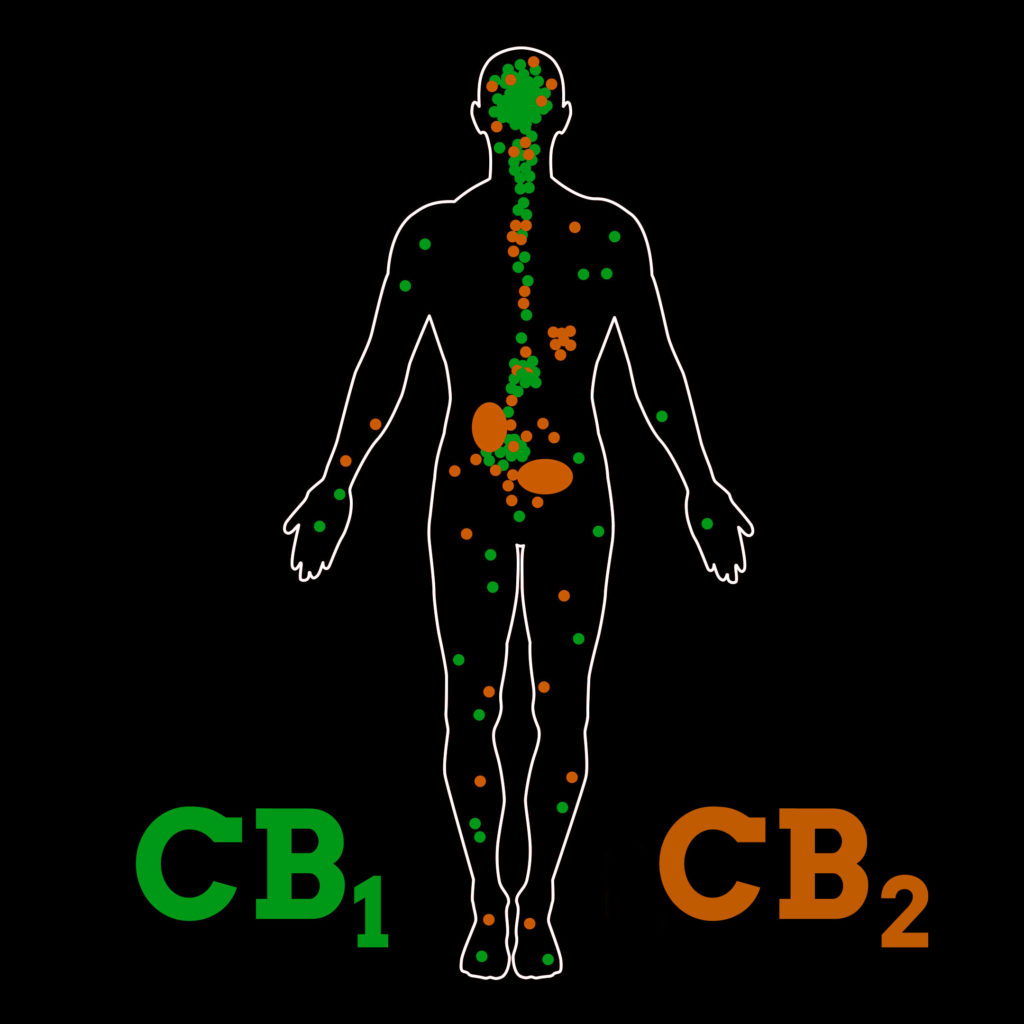
The isomers of THC
Although popularly referred to simply as THC, the THC molecule in question is more properly known as delta-9-tetrahydrocannabinol, or Δ9-THC. There are in fact other forms of THC, of which the best known is Δ8-THC.
These other forms (known as “double bond isomers”) are essentially the same molecule, but differ in one tiny respect. The THC molecule contains a cyclohexane ring – six carbon atoms arranged in a ring, each bonded to two hydrogen atoms. These carbon atoms are linked by single covalent bonds, except for one pair that is linked by a double bond. The position of this double bond determines which of the double bond isomers the molecule is, as well as the extent of its psychoactive effect.
Most of these isomers are greatly understudied, but what little research there is has shown that only Δ9-THC and Δ8-THC act on the CB1-receptor. Activating the CB1-receptor is a rare ability, and is the main cause of the psychoactive nature of cannabis.
Interestingly, Δ8-THC has been shown to have potentially greater ability to stimulate appetite than its more famous counterpart, in one 2004 study on mice. However, it generally seems that its ability to stimulate the CB1-receptor is less than that of Δ9-THC, and for this reason, interest in it has not persisted.
As well as these double bond isomers, THC also has several structural isomers. The difference here is that double bond isomers contain atoms arranged in identical ways but linked together with different bonds, while structural isomers contain the same atoms, but which are arranged differently to give a (sometimes radically) different 3D structure. Some of these structural isomers are very well-known to those with an interest in cannabis – for example, cannabidiol (CBD) and cannabichromene (CBC)!
The importance of THC in medicine
THC, along with several other cannabinoids, binds to special “receptor” sites in the body that are located in the brain, within the major organs, and throughout the cells of the immune system. These “receptors” are specialised proteins that are situated at the presynaptic junctions between neurons (nerve cells). So far, two major receptors associated with cannabinoids have been identified – cannabinoid receptors type I & II, or CB1 and CB2.
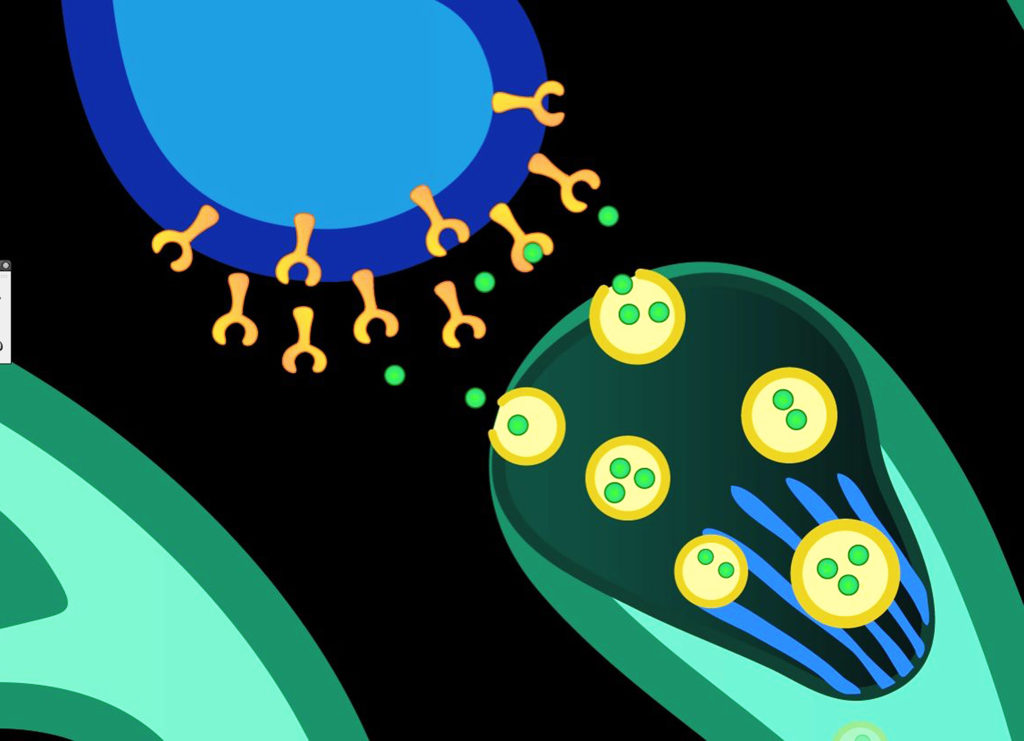
The cannabinoid receptors can be thought of as the “locks” while the cannabinoids are the “keys” that open them. Another term for the keys that fit these specialised protein locks is “ligands”. When a ligand encounters a receptor, it can “bind” to the receptor to create a modified molecule known as the receptor-ligand complex, which has different biochemical properties. The modified molecule may then fulfil a range of different biological functions.
THC is capable of binding to both the CB1 and CB2-receptors. Within the CB2-receptors, the partial binding activity of THC has been associated with crucial regulatory processes such as programmed cell death (apoptosis) and genesis of new cells.
When it binds to the CB1-receptors, which are mostly located in the brain and central nervous system (CNS), the resultant biological activity also takes place within the brain and CNS. This activity within the brain can alter one’s subjective experience of reality, leading to the famous “high” associated with cannabis use.
As well as causing the psychoactive effect, the activity of THC within the brain has been associated with processes related to learning, memory, impulse, emotional regulation, sleep, and appetite. Studying the pharmacological activity of this molecule has proven to be crucial to our understanding of these most fundamental of processes, as well as deepening our insight into the mental illnesses and disorders that can result when these processes go awry.
- Disclaimer:This article is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with your doctor or other licensed medical professional. Do not delay seeking medical advice or disregard medical advice due to something you have read on this website.










Thanks for keeping me updated! Articles like this are perfect reads to keep up with the industry!
It is a go brake down of the molecule that are in the human body that are still the way too early mind and all slow down to move forward to out the year ether white marble breaks down from your body text told, will dwell good Dee.
I live in Silicon Valley and Im surrounded by some of the brightest minds in the world. I have yet to find a scientist or medical professional who can point me towards any literature that contradicts the following;
All cancer cells have a nucleus
All cells with a nucleus use mitochondria as their energy source by manufacturing atp
All cells with a nucleus have cb1 receptors
When ANA or THC bind with a cancer cells cb1 receptor they instruct the nucleus to create ceramide
Ceramide destroys the mitochondria and the cancer cell no longer can survive
Apoptosis
All cancers can be erradicated
Please, anyone, explain to me how Im wrong.
Thanks in advance.
So, why did Bob Marley, of all people, die of cancer? Just asking.
While researching THC Chemistry, I read this report and found the mention of, “CB-2 Receptor Binding”, and it’s relationship to, ” Programmed Cell Death and New Cell Generation”.
I look forward to seeing more on this subject.
Thanks, P.
Yesss! All cancer can be eradicated, but then the pharmacy companies would lose billions of dollars 😬
Thanks. 🙂
Good read.