Terpenoids and terpenes are aromatic compounds that are found in thousands of plant species and are responsible for the various flavours and fragrances of cannabis. We have known about their presence in cannabis for decades, but it is only recently that awareness of their potential therapeutic properties has begun to expand.
Terpenes are a large class of naturally-occurring organic compounds. Terpenes and terpenoids are only slightly different from one another and the terms are commonly used interchangeably.
Terpenes are basic organic hydrocarbons found in many plants, including cannabis. Everyday consumers would better know them as the essential oils that come from the flower’s sticky resin glands.
Terpenoids, also known as isoprenoids, contain more chemical elements (atoms) that have undergone oxidation.
Basically, terpenes are the wet version. Terpenoids are what terpenes become once the cannabis has been dried and cured.
The isoprene rule and its relevance to terpenes
Limonene, which is comprised of two linked isoprene units, can be expressed as (C5H8)2, which equates to C10H16. Several other terpenes also have this structure, but the two isoprene units are arranged differently. Together, they are known as monoterpenes (the mono- prefix refers to the number of complete terpene units; i.e. one terpene unit equals two isoprene units).
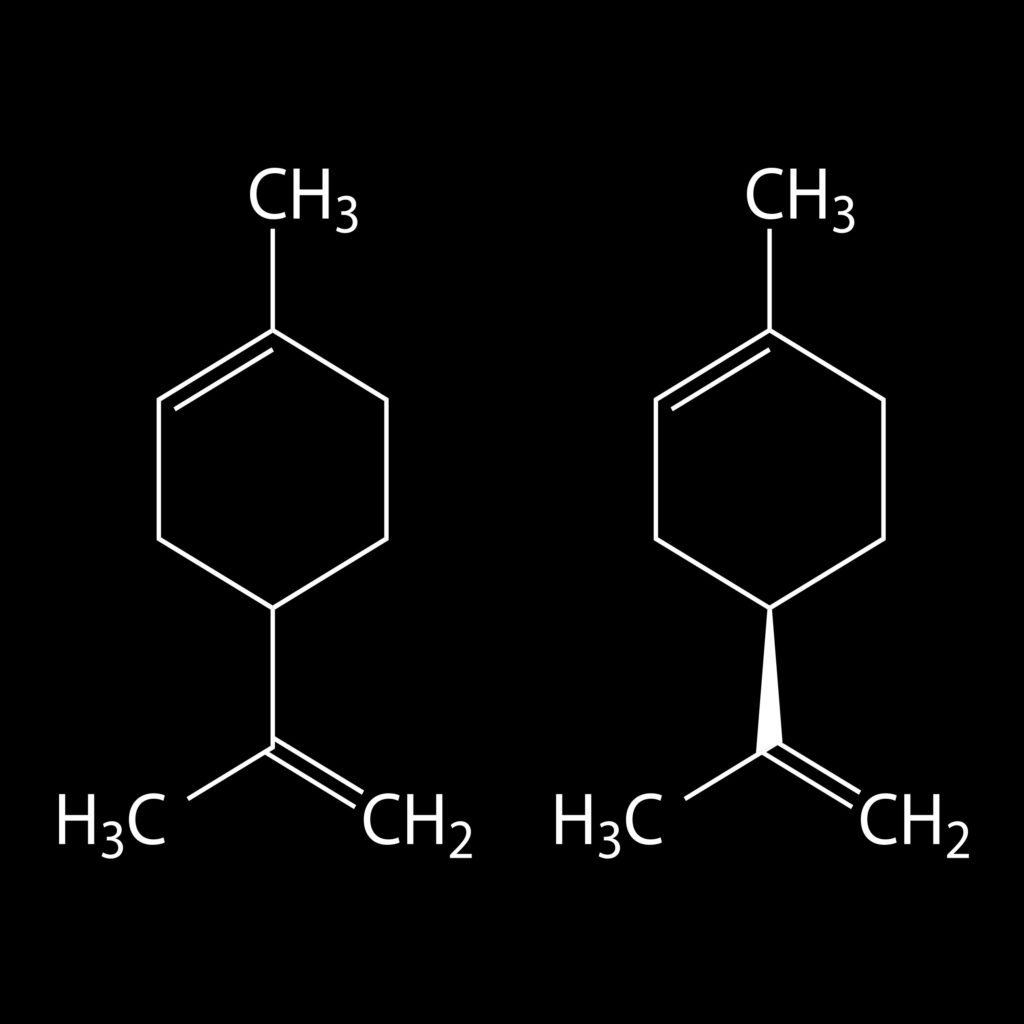
Terpenoids with three linked isoprene units are known as sesquiterpenes (sesqui – meaning 1.5). Those with four are diterpenes, and so on. The formula (C5H8)n, where n is the number of linked isoprene units, is known as the isoprene rule, and is one of nature’s most common building blocks.
The cannabis plant produces cannabinoids through a convoluted series of chemical reactions that is thought to involve terpenes as ‘building blocks’. Cannabinoids are known as terpenophenolic compounds, as they are comprised of terpene blocks attached to phenol (C6H6O) groups. And seeing that terpenes and cannabinoids share the same precursor, an abundance of terpenes is usually a sign of high cannabinoid levels.
Which terpenes are found in cannabis?
It is thought that cannabis contains over 120 terpenes, although many of these are found in trace amounts and may have negligible, if any, effect.
The primary terpenes and terpenoids that have been identified in cannabis are limonene, myrcene, pinene, linalool, eucalyptol, y-terpinene, ß-caryophyllene, caryophyllene oxide, nerolidol, and phytol.
These compounds, unlike cannabinoids (although there are now indications that other plant species do contain some phytocannabinoids), are not unique to cannabis and many are in fact very familiar to us. The 4 most common terpenes in cannabis are:
1. Limonene
Limonene is the monoterpene primarily responsible for the fragrance of citrus fruits — specifically, the D-isomer. D-limonene smells strongly orange-fragranced in its isolated form. It is widely used as a flavour additive in food production and an aroma compound in perfumery.
What medicinal uses might limonene have?
Limonene is used in alternative medicine, due to its observed ability to reduce heartburn and gastric acid reflux.
Research has shown that limonene may be effective for both chemoprevention and chemotherapy of cancer. At least, that is the case with this study on its effects of treating rodent mammary, lungs and stomach cancers.
Another very recent study (2018) verified this again regarding lung cancer in mice. It showed that limonene can promote autophagy, which triggers the death of lung cancer cells.
As well as this, limonene is used as a natural, renewable solvent in cleaning products, due to its ability to dissolve oils (it can dissolve even petroleum in about half an hour in the right conditions!) and other lipids. It is even capable of stripping paint and is considered an effective substitute for turpentine. Limonene must be handled with care by humans, as in high concentrations it can act as an irritant.
D-limonene is now also added to cannabis extracts as a flavour enhancer, as many of the existing terpenes are lost in processing.
2. Myrcene
Myrcene is another monoterpene, and is the most abundant terpene found in cannabis, making up over 60% of the essential oil of some strains. It is also found in bay leaves, wild thyme, hops, ylang-ylang, lemongrass and verbena.
Myrcene is responsible for the ‘green hop aroma’ found in dry-hopped beers (beers that have hops added post-fermentation at low temperatures to enhance their ‘hoppy’ flavour). The aroma itself is described as resinous, herbaceous, and slightly metallic—and very pungent at high concentrations.
Another plant that contains significant myrcene is Myrcia sphaerocarpa (Myrcia is the genus for which myrcene is named). It is a small shrub with astringent leaves and roots, which is native to Brazil and has long been in use there as a treatment for diarrhoea, diabetes, and hypertension.

What medicinal uses might myrcene have?
Myrcene has been demonstrated to produce analgesic effects in laboratory testing on rats. Used with limonene, along with the terpenoid citral (found in many citrus fruits, lemon myrtle, lemongrass, and lemon verbena) it has also been found to exert sedative and motor relaxant effects in mice.
3. Pinene
Pinene is another monoterpene, which occurs naturally as two isomers (molecules with the same chemical formula but different structures). These isomers are known as α-pinene and ß-pinene. They are usually sourced from turpentine (produced by dry distillation of coniferous wood) and make up 58-65% and around 30% of its total volume, respectively.
Both α- and ß-pinene are found in pines and other conifers, as well as in Salvia (sage), Artemisia (sagebrush), and Eucalyptus. A-pinene is also found in olive, rosemary, sassafras, and bergamot. It is the most widespread naturally-occurring terpene. B-pinene is also found in hops and cumin.
A-pinene is known for its inhibitory effect on root growth in many plant species, thought to occur through the production of reactive oxygen species that cause oxidative stress within the root system. It is believed that many plant species exude it from their leaves as a natural herbicide, preventing other plants from competing against them for resources.
What medicinal uses might pinene have?
In an animal study, pinene exhibited anti-Infectious Bronchitis Virus properties. Another study demonstrated that pinene has a bactericidal effect in methicillin-resistant Staphylococcus aureus (MRSA), and an antifungal effect against Candida albicans.
4. Linalool
Linalool is a monoterpenoid with the chemical formula C10H18O It is found in hundreds of plant species including mint, laurel, cinnamon, birch, and some citrus. Linalool is a chiral molecule, meaning that it has two enantiomers, or two isomers that are non-superimposable mirror images.

The ‘left’ enantiomer is known as S-linalool and is found in coriander, palmarosa grass, and sweet orange. It has a sweet, floral scent. The ‘right’ enantiomer is known as R-linalool and is found in lavender, basil and bay laurel. It has a woody, astringent aroma.
What medicinal uses might linalool have?
Linalool’s main medicinal function is as an anxiolytic—an anxiety-reducing drug. Lavender has been in use as a calmative for thousands of years, and recent tests on rats have borne out its effects to modulate locomotion and motor movements.

Other noteworthy terpenes & terpenoids in cannabis
- Eucalyptol: Eucalyptol, a monoterpenoid, is abundant in nature. As well as in cannabis, it is found in eucalyptus, tea tree, bay leaves, basil, and sage. It is well-known for its antiseptic, antibacterial and anti-inflammatory properties.
- G-terpinene: G-terpinene, a monoterpene found in various citrus fruits and herbs such as oregano and marjoram, has antioxidant properties.
- Phytol: Phytol, a diterpenoid, is used by insects as a deterrent to predators, and is also used in various household products such as detergents and soaps.
- B-caryophyllene: B-caryophyllene is a sesquiterpene found in cloves, rosemary and hops. It exhibits anti-inflammatory effects and has been demonstrated to act as a selective agonist of the CB2-receptor (no other terpenes or terpenoids have been found to affect the CB receptors). Caryophyllene oxide is the substance in cannabis that is identifiable by drug-sniffing dogs.
- Nerolidol: Nerolidol, present in neroli, ginger and jasmine, is a sesquiterpenoid with a fresh, woody scent. It is currently being investigated both as a facilitator for transdermal delivery of drugs (due to its ability to penetrate the skin) and as an inhibitor of Leishmania protozoa.
- Guaiol: Guaiol is a sesquiterpenoid found in cypress and Guaiacum (a genus of five slow-growing shrubs and trees native to the tropical Americas). Guaiacum itself has been in use as a treatment for relief of upper back musculoskeletal pain and spasm, and for syphilis for centuries. Guaiacum has also been used to make coughs more productive (by loosening mucus in the airways).
- Eudesmol: Eudesmol, another sesquiterpenoid, is used as a fixative in perfumery, while trans–ß-farnesene acts as a natural insecticide in many plant species, including potatoes.
Terpene differences in sativa and indica strains
Of course, terpene and terpenoids concentration will vary between strains and between related individual plants. It can even vary between two clones of the same plant, if subject to different environmental conditions while growing.
Understanding which terpenes/terpenoids a strain contains can be important, as the type and concentration of each will affect the high it generates. For example, here’s a look into the main difference between dominant terpenes in indica type strains and sativa type strains:
Indica
Indica strains seem to be dominated by the presence of β-myrcene, with α-pinene or limonene being present in slightly lower concentration. Having a high concentration of myrcene, these strains will likely have a relaxing, sedative effect.
Sativa
The terpene profiles of sativa type strains aren’t as reliably characterized, as their profiles aren’t as predictable. Just like indica strains, they can present β-myrcene as the dominant terpenoid, with α-pinene or α-terpinolene abundancy coming in second highest. But α-pinene or α-terpinolene may also be the dominant terpene in some cases.
If it’s the latter, the strain will probably produce more of the typical euphoric, uplifting high.
However, here’s where it gets even trickier. While yes, indica strains are known to often have a high concentration of myrcene, researchers have noted that isn’t always the case. They’ve found that plants of the wide-leafed Afghani Cannabis indica type are more likely to contain high ratios of guaiol, isomers of eudesmol, and other unidentified compounds.
And plants of the narrow-leafed C. indica type that is indigenous to the valleys of the Himalayas are higher in trans–ß-farnesene.
The health benefits of these terpenes are not fully understood, but they may contribute to the differences in medicinal properties found between the different subtypes of cannabis. As our understanding grows, so too will knowledge of how best to develop and utilise new medical strains.
- Disclaimer:This article is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with your doctor or other licensed medical professional. Do not delay seeking medical advice or disregard medical advice due to something you have read on this website.











This is such a detailed blog, medical benefits of each one of them! Thank you for sharing this. It helps in deciding which natural terpene for cannabis to buy.
The interesting point of this article is the isoprene rule to determine the chemical structure of terpenes. I have also got information on the medicinal uses of terpenes. My physician has recommended using CB2 hemp seed oil for the treatment of insomnia. It is a problem which has occurred due to the workload and late-night work. I am happy that I have to use this valuable oil and hope it will cure my sleep disorder very soon.
Great stuff on terpene and cannabis and its medicinal values. I came to know several health benefits about the terpenes. I am a user of the CB2 hemp oil and after reading this article I believe the addition of the terpenes can aid further improvement in pain management. Terpenes are good analgesic agents and I have to purchase oil with a combination of terpenes with CBD for the treatment of gout.
Cant agree that phytol and nerolidol are primary terpenes. phytol is uncommon in cannabis and caryophyllene oxide is just an oxidation product of beta caryophyllene, its not even synthesized by the plant. You will find the oxide on photo or heat degraded cannabis(not the fresh harvest). trans beta farnesene is a common terpene found in Gelato, sunset sherbert, some cookie(thin mint) phenos and in LA confidential. In LA confidential it is the most abundant terpene. Many cannabis labs dont even assay beta farnesene in their terpene analysis.
Great article. We should collaborate on some terpene R&D. We provide strain profiles and isolates.
Great article, lots of informative material,Very nice work , thank you for the information
Thanks,
Terpenes and Testing Magazine
Great article – I have been using terps for about 2 years now, I buy them from Kleanterpenes.com in the Aroma section. I use alpha pinene, limonene and citral in an aromatherapy diffuser in the morning to wake up (i’ll use an e-pen mixed with propylene glycol if I’m especially tired). I use myrcene, linalool and one of terpineol, terpinene or terpinolene at nighttime before I go to bed and it keeps me out all night. I add nerolidol if I’m having a lot of trouble sleeping, or maybe I add linalool and myrcene to a sock and put it under the covers. All this said, these are concentrated aromatics and certain ones can irritate the skin, or the eyes, or like linalool is therapeutically anxiolytic at 1ppm in the air but is toxic at 20ppm. When I first started I took too much and turned my right arm red for 2 days with a rash. Not to mess around with, don’t drink it and don’t take a bath in it. Pinene as another example is only shipped ground because it is as flammable as pure alcohol. Don’t light a match around it, but breathing small amounts improves lung function and can even kill the most stubborn lung infection. These same compounds have anti-cancer activity, so the person with the herb garden in King. Sage, Rosemary, Dill, Thyme, etc all have these compounds. I currently mix custom flavors for people to add to bong water or diffusers for non-cannabis people. Long live terpenes!
would like to know the modern methods of extraction and separation of terpenoids
What is the work of terpenoid in Annona muticata
Hello Emmanuella,
Thank you for your comment. I’m afraid we cannot answer that question as the plant you refer to (I think you mean Annona muricata?) is not covered in this article, and is not a member of the cannabis family. Sorry we can’t be more help!
With best wishes,
Scarlet
It provides a clear cut view about terpenoids.
I have visited the Greek island of Kefaloni three times and on each visit have notice a strong ‘cannabis’ type smell along the roadside. On my last visit I manage to identify the plant that was producing the aroma. It was a lush green and had clumps or clusters of what I imagine were seed pods of some sort. Unfortunately I couldn’t keep my sample.
Have you any idea what this plant may be? Are hops, tea and cannabis related by the same aromatic compounds and is this compound found naturally in other species?
You should say what terpenes are useful for what type of effects?
Limonene for up, Myrcene for couchlock for example.
And there are 140 terepenes found in Cannabis that they have ID’ed so far, email me and I will send you the list.
I tried the top 12 terpenes one at a time, with 25mg pure THC with Rob Clarke.
You have just scratched the surface of terpenes.
Hey David,
I would love to learn more about all the terpenes in cannabis and your experiences. Could you email me the list, when you have a second. I would love to share this info with my patients.
Thanks in advance.
Chris
chris@greenwaydoctors.com
David- I would love to hear more about what you are doing and have done with profiling terpenes….
n.netherland@medicann.com
Hi David,
If you’re willing to share, I’d also love to hear more about what you’re up to.
Thanks,
Dan
I would love to hear more about what you are doing and have done with profiling terpenes
I’m a lab manager at a cannabis testing facility. I would find the list of terpenes to be extremely useful for our clients.
Please email me if you get a chance. I sure appreciate it!
Hi could you send me the list. I am a retired nurse this yr. My brother has lung cancer. I need info on the terpenes that destroy tumors. I don’t know much about terpene in Hemp oil and type of terpenes. I am desperate!
I have made the following below for many people and they have had very good results. For lung cancer specifically Suppositories is the most effective as it is the quickest most direct route through Inferior Vena Cava vein.
(The story actually started in 1978 when a man living in the Gold Coast survived his cancer by drinking the boiled extract of pawpaw leaves. He was the one who first suggested to try the pawpaw leaves as a possible cure for cancer 14 years ago.
He was dying of lung cancer when he decided to take the alternative therapy and cleared his lungs with any signs of cancer. The amazing remedy was also tried by a banana grower suffering from a gall bladder cancer. After five weeks, he showed no trace of cancer whatsoever.)
Best Wishes
Morning Dose
Cannabinoids: CBD 100 mg,THC 50 mg, CBG 250mg
Terpenes: Alpha Pinene 45, Beta Pinene 100 mg, Eucalyptol 10mg
Polyphenols: Black Cumin Seed 1 tsp, Bromelain 1000mg, Bee propolis extract 60% 200mg,Cleaver herb 400mg,Cordyceps 40% 500mg, Curcumin water soluble 95% 500mg, Ganoderma Lucidem 500mg, Guggul extract 2.5% Guggulsterones 200mg, Shilajit extract 40% fulvic 200mg, 95% Quercetin 1000, Amygdalin 98% 250mg, Zinc gluconat 125mg, Astaxanthin 10% extract 200mg, Sage extract 20:1 scaleorl 450mg, Naringin 98% 100mg, Aescin Horse Chestnut Extract 100mg, Alpha Lipoic ACID 200mg, Carica 5:1 Fermented Papaya 400mg, Bitter Melon 500mg, Holy Basil Leaf 1000mg, Papaya Leaf Extract 10:1 500mg, Sunflower Lecithin 1800mg, Saffron Extract 100mg, Brocolli Sprout Extract .4% 250mg, Boswellia 300mg, AKBA 20% Boswellic Acid 50mg
Evening Dose
Cannabinoids: CBD 50 mg,THC 50 mg, CBG 250mg
Terpenes: Eugenol 15mg, Terpineol 100mg, Terpinolene 150mg, Nerolidol 50mg
Polyphenols: Black Cumin Seed 1 tsp, Bromelain 1000mg, Bee propolis extract 60% 200mg, Cleaver herb 400mg, Cordyceps 40% 250mg, Curcumin water soluble 95% 500mg, Guggul extract 2.5% Guggulsterones 200mg, Shilajit extract 40% fulvic 200mg, Amygdalin 98% 250mg, Astaxanthin 10% extract 200mg, Alpha Lipoic ACID 200mg, Carica 5:1 Fermented Papaya 400mg, Bitter Melon 500mg, Holy Basil Leaf 1000mg, Papaya Leaf Extract 10:1 500mg, Sunflower Lecithin 1800mg, Saffron Extract 100mg, Brocolli Sprout Extract .4% 250mg, Boswellia 300mg, AKBA 20% Boswellic Acid 50mg
Hey David, or anyone else that has received the information. Do you still have the list to share for the Terpene effects and other information that you would like to share.
miltonares@gmail
good literature
Very informative and useful
It’s awesome to learn that not all cannabis will get you stoned.This entourage effect is a revolutionary scientific solution for procuring health benefits from cannabis.
We are a bunch of volunteers and starting a new scheme
in our community. Your site provided us with useful information
to work on. You’ve done an impressive process and
our entire community shall be thankful to you.
A very informative and interesting article. Always a pleasure to read about positive attitudes towards the properties of cannabis, and in the way it may be able to benefit the use of cannabis for medicinal purposes.
Great article about terpenes & terpenoids, thanks for sharing this!