Simply put, the endocannabinoid system is an essential biological network similar to the central nervous system, in that it is vital to the healthy functioning of bodies and it is not restricted to one organ or body part. Amazingly, it was discovered thanks to cannabis use and users, so don’t believe anyone saying we never contribute anything to society!
What is the endocannabinoid system?
The endocannabinoid system is, scientifically speaking, made up of neurons, endocannabinoids and cannabinoid receptors. This article is going to break this statement down into simpler terms, as this is fascinating stuff, but requires some explanation for pretty much everyone who hasn’t studied neuroscience (this includes the writer!).
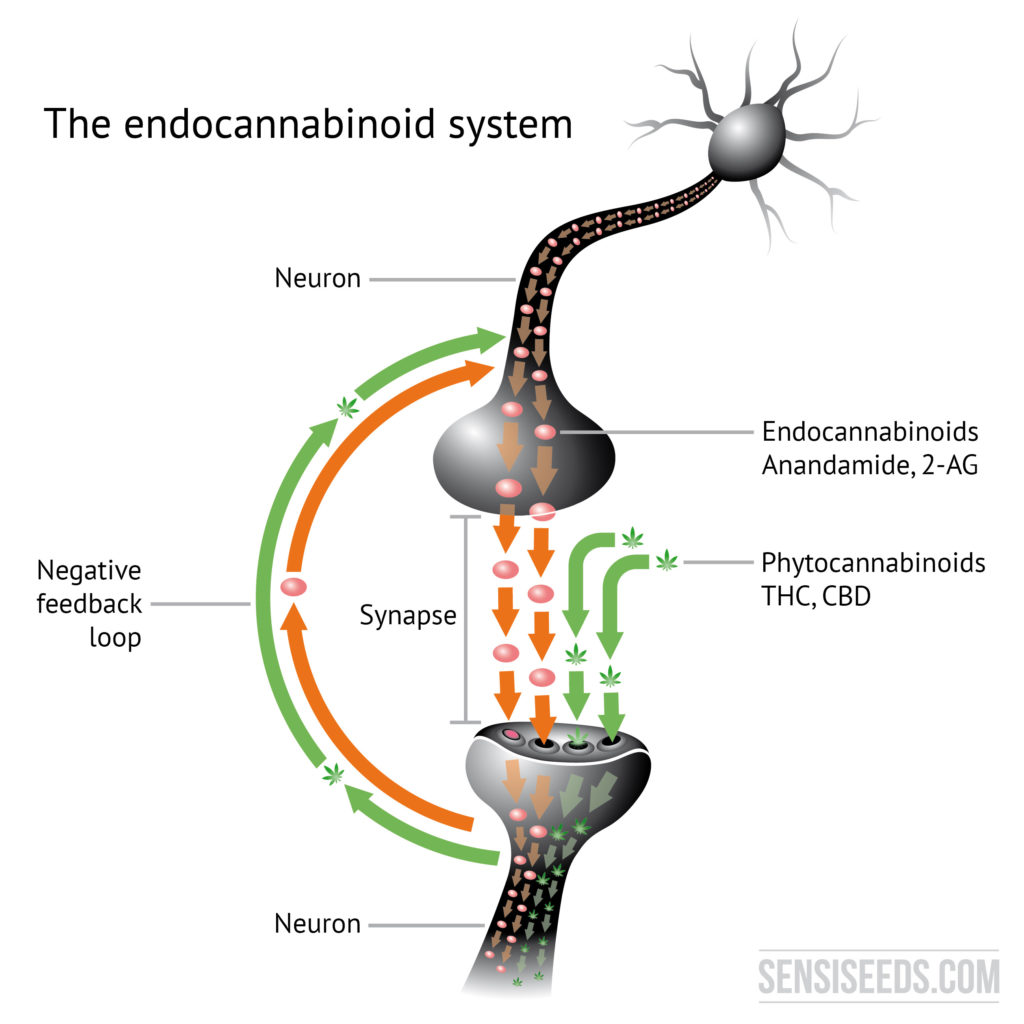
There are nerve cells called neurons throughout the brain and body which are linked together by neurotransmitters. These neurotransmitters are molecules called agonists that move from one neuron to another through the minute space between them, which is called the synapse.
The agonists plug into neural receptors, causing a chain reaction. In the case of the endocannabinoid system, these receptors are called CB1 (Cannabinoid receptor 1) and CB2 (Cannabinoid receptor 2).
CB1 receptors are mainly found in the brain, with some in the liver, lungs, and kidneys. CB2 receptors are found throughout the body. There are more cannabinoid receptors in the brain than any other type of neural receptor.
A common analogy is that the agonists are keys and the receptors are locks. However, you can also think of the neurons as being like Lego blocks, with the studs* as agonists and the tubes* as receptors. The endocannabinoid system is a structure. It can only work if the blocks fit together. And as you’ll see, it’s essential that it does work!
Endocannabinoid system sends signals within the brain and around the body
Cannabinoids transmit signals from one neuron to another
CB1 = cannabinoid receptor 1. Location: Mostly brain
CB2 = cannabinoid receptor 2. Location: Body
How does the endocannabinoid system work?
The endocannabinoid system is activated by (surprise, surprise) cannabinoids. The cannabinoids naturally produced by the body, which are known as endocannabinoids; and the cannabinoids found in cannabis, which are known as phytocannabinoids.
The key and lock analogy mentioned above is based upon the CB1 and CB2 receptors only being activated by cannabinoids, not any other type of agonist molecule. The cannabinoid ‘keys’ are the only ones that will fit the receptor ‘locks’.
Phyto = prefix meaning a plant or plants
Endo = prefix meaning within or inside
Phytocannabinoids, also called classic cannabinoids, come from plants
Endocannabinoids come from inside the body
The CB1 receptors are activated by THC (tetrahydrocannabinol) – so when we talk about the ‘headrush’ effect caused by sativa-dominant, THC-heavy strains, there’s a literal quality to that statement!
The CB2 receptors are activated by CBD (cannabidiol), which is not psychoactive and more associated with cannabis strains (and pure CBD products) that give a relaxing, body-centric effect. This makes the location of, and difference between, the two receptors easy to remember!
CB1 = THC = head
CB2 = CBD = body
What is the endocannabinoid system for?
The endocannabinoid system regulates the body’s systems to maintain homeostasis: the state of balance necessary for healthy function. Homeostasis can be thought of as the narrow range of states within which bodies work as they should.
For example, the blood sugar levels, internal temperature, pH levels of blood, regulation of the amount of water and minerals in the body, and the removal of metabolic waste, are all governed by homeostatic processes.
A simple analogy is to think of the body as a house, and the endocannabinoid system as the caretaker inside.
If the house is too hot, the caretaker opens the windows or turns on the air conditioning. If the house is too cold, the caretaker closes the windows and turns on the heating. If the house becomes dirty, the caretaker cleans it, but also knows when to stop cleaning it – you wouldn’t throw away all the rubbish and then start on the furniture.
Body = house
Endocannabinoid system = caretaker
Cannabinoids = messages the caretaker receives about what needs to be done

Most agonists only travel in one direction. Cannabinoids are unusual in that they can travel both ways between neurons. This is known as a negative feedback loop. It is what makes the ECS such an essential system for lifeforms. It tells the body when to begin a process (for example, sweating to cool down) but also when to stop it (otherwise we’d all be sweating constantly).
Bodies constantly make their own cannabinoids (endocannabinoids) to interact with their endocannabinoid system, ensuring that homeostasis continues.
If not enough endocannabinoids are created, it is thought that clinical endocannabinoid deficiency may occur. It is also thought that this can be treated by introducing phytocannabinoids, something that humanity has been doing with varying degrees of therapeutic success since before recorded history.
The reason cannabis can treat so many different conditions is that the endocannabinoid system is spread throughout the body and responsible for the correct functioning of so many different parts and aspects of it.
Are humans the only ones with endocannabinoid systems?
Absolutely not! The reason this article refers to ‘the body’ rather than ‘the human body’ is that more creatures on this planet have an ECS than not.
All vertebrates (creatures with a backbone) and invertebrates (creatures without a backbone) have one. This explains why CBD products are having such success when used on pets, and have the potential to treat a virtually unlimited number of species.
There are a few species that don’t have one, such as sea sponges, nematode worms and anemones, since their evolution diverged from ours so long ago. The earliest lifeform known to have cannabinoid receptors is the sea-squirt.
This primitive tube-shaped creature evolved more than 600 million years ago, and vomits up its internal organs as a self-defence move (rather like very drunk people avoiding pub brawls). There is even a type of slime mould that “possesses a rudimentary endocannabinoid system”.
When was the endocannabinoid system discovered?
You might think that, since the ECS is so ancient, so vital, and so common in lifeforms, it would have been discovered long ago. You would be wrong. The endocannabinoid system was only confirmed in the form that we know it today (CB1 and CB2 receptors, triggered by two known endocannabinoids) in 1995 (raise your hand if you’re older than this crucial discovery!).
1940 – CBD first isolated
1963 – CBD first synthesised
1964 – THC first synthesised
1988 – CB1 identified (in rats)
1991 – CB1 in humans successfully cloned
1992 – Anandamide, the first endocannabinoid, discovered in human brain
1993 – CB2 identified in humans and successfully cloned
1995 – 2-AG, the second endocannabinoid, discovered
The phytocannabinoid CBD was first isolated in 1940, but not until 1963 did Professor Mechoulam and his team discover its chemical structure and successfully synthesize it. Their feat was replicated with THC a year later.
In 1988, the first cannabis receptor was identified; in 1993, the second. The first endocannabinoid, named anandamide, was only discovered in 1992 and the second, known as 2-AG (because only a handful of highly skilled individuals can easily pronounce 2-arachidonoylglycerol) followed in 1995.
How was the endocannabinoid system discovered?
The only reason that we now know about this incredible system is that prior to the late 1980s, research funded by the National Institute of Drug Abuse was taking place which was supposed to discredit cannabis users.
The potential to treat hundreds of afflictions has been unlocked because for thousands of years, humans have enjoyed the altered states produced by cannabis and refused to give them up just because its legal status was changed.
However, this does not mean humans are ‘meant’ to use cannabis (despite what Bill Hicks said). It would be wrong to say “this proves we were designed to use cannabis” unless your belief system incorporates beings who 1. designed humans, 2. have some kind of master plan which involves us using cannabis, and 3. have either given up on making sure we’re all able to do this without fear of imprisonment, or are somehow capable of being thwarted by us not sticking to their plan.
What it does prove is that nature is amazing, humans are not as different to other lifeforms as previously believed, cannabis has more medicinal potential than any other plant on Earth, and now we know why
In fact, the last word should go to Professor Mechoulam, who sums up this point with simple eloquence:
“By using a plant that has been around for thousands of years, we discovered a new physiological system of immense importance… We wouldn’t have been able to get there if we had not looked at the plant.”
Did you learn something new from this article? Is there an angle on the endocannabinoid system that was not covered, and that you still have questions about? Are you a researcher with a contribution or correction on any of the points? Let us know in the comments!
*these are the official names for the parts of Lego pieces. I checked.
- Disclaimer:This article is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with your doctor or other licensed medical professional. Do not delay seeking medical advice or disregard medical advice due to something you have read on this website.










Wow what an incredible article, this is such an interesting piece of knowledge about the body, “the cleaner knows when to stop when they know to” basically means the more you take care of your health in whatever, mental, emotional, physical and spiritual way that is honest with yourself. And homeostasis will optimise, using a small does or a delayed dose of weed definitely has profound, internal kinaesthetic body awareness, making things like balance, “naturalising” yourself into ambidextrous states/actions, flexibility and tensions easier.
Sports performance may increase in some way (a clear mind or meditative state helps)
I don’t personally use THC or CBD. I did grow up on it as a child. Now, as an adult it causes me to feel nausea and sometimes throw up. I enjoyed reading several of your articles. Even though my parents live in different states they both have their medical cards. Family and friends all tell me that I would feel so much better if I did partake. Maybe you could tell me how to get over the hours of agony I feel when I do crack under pressure. As I suffer with pain and mental illness. I quit the pain killers but I still take mood stabilizers. I have for a long time wanted to stop taking medicine. Which I am afraid to stop taking due to having a break down many times before, I just hate taking medicine. Thank you for all your articles. So, anyways if anyone could suggest a remedy other than take a smaller puff. Tried it and still I can’t function for several hours.
Hi Darrah,
Thank you for your comment and your positive feedback about our articles! I wish I could help you with your situation, but as we are not a medical practice, I can’t give you any advice other than to consult a registered healthcare provider – and to say that cannabis doesn’t work for everyone, it’s not a magic one-size-fits-all remedy and we still don’t understand enough about how it interacts with the body to be able to explain all the effects it has.
If you do decide to speak with a healthcare provider, we have a few more articles that may be of interest to you if you haven’t already seen them. This article about the potential benefits of medicinal cannabis might be useful for you to show your healthcare provider if they are not familiar with it.
You may also find it helpful to contact a support group for medicinal cannabis patients. In the UK there is the United Patients Alliance, and throughout much of the rest of the world there is NORML, who should be able to put you in touch with a group in your area (search United Patients Alliance or NORML followed by your area name).
These are our pages on medicinal cannabis and medicinal cannabis strains, which you might also find interesting.
With best wishes,
Scarlet
Isn’t the natural world amazing,I have always suggested a good friend of mine who is at times unable to describe the illness I will call it & not so long ago was diagnosed with type 2 diabetes that she should try some cannabis. Even tho it was said to calm her down most times but when she did consider the thought I used the medical theories we hear throughout the media to convince her that it actually is proved in science,now all I have to do is get her to read your blog lol.
I know I’m,fantastic stuff
Hi Paul,
Thank you for your comment and positive feedback. I hope you and your friend continue to enjoy the blog! She might find this post on cannabis and diabetes interesting.
With best wishes,
Scarlet
Thank You very informative and clearly and simply explained, Congrats,
Hi Alejandro,
Thank you so much for your positive feedback, this is exactly what I was aiming to achieve. I hope you continue to enjoy the blog!
With best wishes,
Scarlet
Quick question.
Is whole plant far better that all the snake oils and pharmaceutical?
.
Doctors are trying to tell me that pharmaceutical products are just as effective.
.
I don’t believe what they say is true.
Hi John,
Thanks for your comment. As Sensi Seeds is not a medical agency or practitioner, we cannot give any kind of medical advice other than to consult your registered healthcare professional. However, you will probably find this article and video on what is known as the Entourage Effect useful. I’m not sure what you mean by ‘snake oils’ though!
With best wishes,
Scarlet
the term snake oil is usually used to say a substance with no real (proven) medicinal value sold as a remedy for all diseases. so a synonym for a so called fixes all wonder potion/medicine but actually it’s fake/none working fake medicine see also Wikipedia: https://en.wikipedia.org/wiki/Snake_oil
Hi Simon,
I think you’re trying to reply to John (comment above)?
With best wishes,
Scarlet
What’s the different in cbd and cbd?
Hi Dianne,
Thanks for your question. I think you are asking about the difference between cbd and cbn, or possibly cbd and thc? We have a series of articles about the different cannabinoids which should hopefully answer your question.
article on CBD
article on CBDA
article on THC
article on THCA
article on CBN
I hope these are useful 🙂
With best wishes,
Scarlet
good one .